reference:
KFA Van Damme, L Hoste, J Declercq, E De Leeuw, B Maes, L Martens, R Colman, R Browaeys, C Bosteels, S Verwaerde, N Vermeulen, S Lameire, N Debeuf, J Deckers, P Stordeur, P Depuydt, E Van Braeckel, L Vandekerckhove, M Guilliams, STT Schetters, F Haerynck, SJ Tavernier, BN Lambrecht.
A Complement Atlas identifies interleukin 6 dependent alternative pathway dysregulation as a key druggable feature of COVID-19.
COVID-19 Complement Atlas - introduction
About
Welcome to the online portal of the Complement Atlas, a resource which allows exploration of diverse datasets on complement dysregulation in COVID-19. This Atlas is the outcome of a collaborative effort by researchers at Vlaams Instituut voor Biotechnologie (VIB), Ghent University, Ghent University Hospital, as well as numerous health care workers across Belgium who contributed to randomized clinical trials conducted during the 2020 COVID-19 pandemic. Collectively, these data provide new insights on how the complement system, a key component of the immune system meant to defend against pathogens, contributes to lung injury in COVID-19. These findings not only open up novel therapeutic avenues in COVID-19, but also might hold promise for addressing complement dysregulation in other human diseases.
The complement system
The complement cascade is an indispensable and multifunctional arm of the innate immune system. It contributes to pathogen recognition and phagocytotic clearance, recruits and activates immune cells, and induces direct pathogen or cell lysis via the membrane attack complex (C5b-9, MAC). Complement responses can be initiated via three pathways (see Figure below). The classical and lectin pathways are triggered by immune complexes and pathogen recognition molecules respectively, leading to cleavage of C4 and C2. The spontaneous hydrolysis of C3 triggers further activation of native C3 to set off the alternative pathway. All modes of complement activation converge on the proteolysis of C3 and C5, generating the potent pro-inflammatory peptides C3a and C5a, while C5b initiates MAC formation.
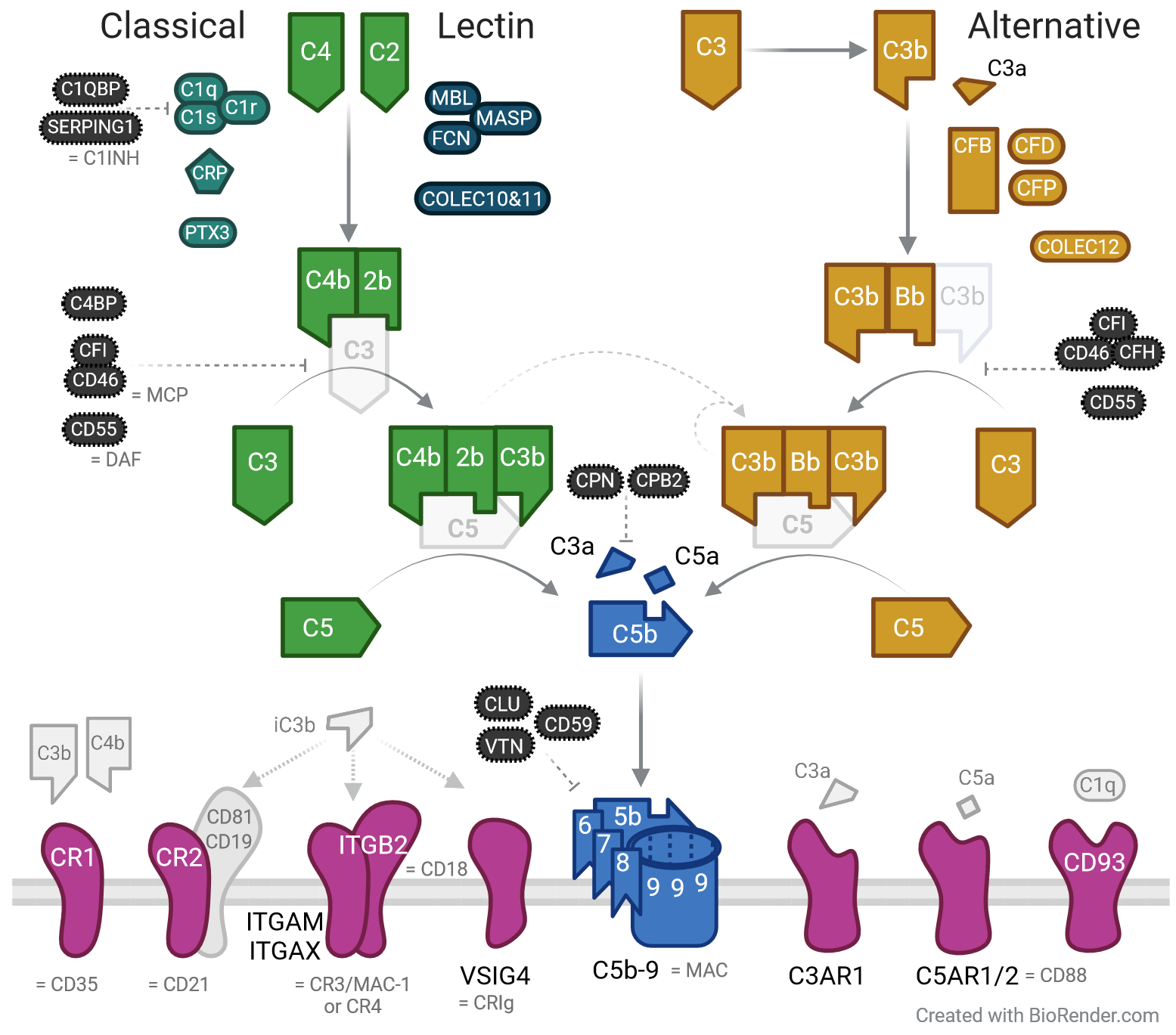
To prevent inappropriate immune activation and collateral damage to tissues, complement responses are tightly controlled under homeostatic conditions and protective immune responses. The key importance of complement and its regulation is best illustrated by genetic complement deficiencies, which lead to immunodeficiency, auto-immunity, endothelial damage and/or kidney injury. In addition to these inborn disorders, dysregulation of the complement system has been reported across a spectrum of infectious diseases, including those caused by emerging coronaviruses.
Scientific abstract
Improvements in COVID-19 treatments, especially for the critically ill, require deeper understanding of the mechanisms driving disease pathology. The complement system is a crucial component of innate host defense, but can also contribute to tissue injury. Although all complement pathways have been implicated in COVID-19 pathogenesis, the upstream drivers and downstream effects on tissue injury remain poorly defined. We demonstrate that complement activation is primarily mediated by the alternative pathway, and we provide a comprehensive atlas of the complement alterations around the time of respiratory deterioration. Proteomic and single-cell sequencing mapping across cell types and tissues reveals a division of labor between lung epithelial, stromal, and myeloid cells in complement production, in addition to liver-derived factors. We identify IL-6 and STAT1/3 signaling as an upstream driver of complement responses, linking complement dysregulation to approved COVID-19 therapies. Furthermore, an exploratory proteomic study indicates that inhibition of complement C5 decreases epithelial damage and markers of disease severity. Collectively, these results support complement dysregulation as a key druggable feature of COVID-19.
COVID-19 Complement Atlas
COVID-19 Complement Atlas
COVID-19 Complement Atlas
COVID-19 Complement Atlas
reference:
KFA Van Damme, L Hoste, J Declercq, E De Leeuw, B Maes, L Martens, R Colman, R Browaeys, C Bosteels, S Verwaerde, N Vermeulen, S Lameire, N Debeuf, J Deckers, P Stordeur, P Depuydt, E Van Braeckel, L Vandekerckhove, M Guilliams, STT Schetters, F Haerynck, SJ Tavernier, BN Lambrecht.
A Complement Atlas identifies interleukin 6 dependent alternative pathway dysregulation as a key druggable feature of COVID-19.
COVID-19 Complement Atlas
Reused publicly available datasets:
reference:
KFA Van Damme, L Hoste, J Declercq, E De Leeuw, B Maes, L Martens, R Colman, R Browaeys, C Bosteels, S Verwaerde, N Vermeulen, S Lameire, N Debeuf, J Deckers, P Stordeur, P Depuydt, E Van Braeckel, L Vandekerckhove, M Guilliams, STT Schetters, F Haerynck, SJ Tavernier, BN Lambrecht.
A Complement Atlas identifies interleukin 6 dependent alternative pathway dysregulation as a key druggable feature of COVID-19.
COVID-19 Complement Atlas - data and additional reading
Randomized clinical trials
Datasets
Download complement level dataset.Download sC5b-9 levels upon anti-C5 treatment.
Download complement activity dataset.
Download proteomics dataset (Olink Explore 3072).
Download README for the above datasets.
Funding
Acknowledgements
Contact information
If you have any questions or suggestions, please contact us at karel.vandamme@ugent.be or bart.lambrecht@ugent.be.


